Success for novel DED drop
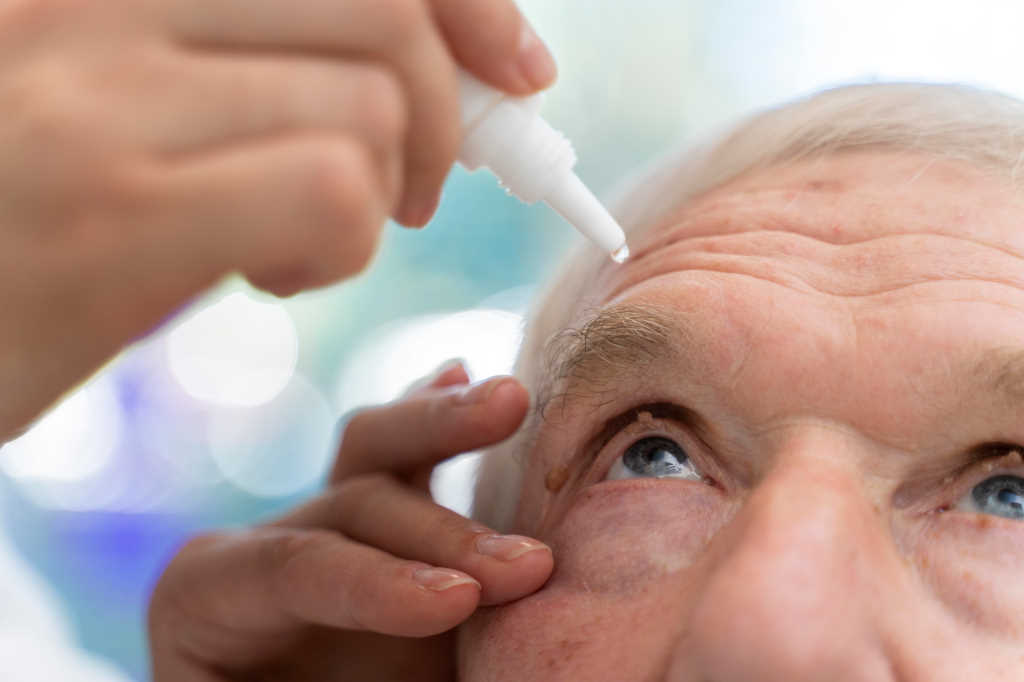
A novel cyclosporine drop formulation demonstrated significant improvement in corneal fluorescein staining (CFS) and in modified Symptom Assessment in Dry Eye (mSANDE) scores in patients with dry eye disease (DED), which was uncontrolled on Restasis (0.05% cyclosporine) therapy.
India’s Sun Pharmaceutical’s Cequa uses nanomicellar (‘Ncell’) technology, designed to deliver 0.09% cyclosporin to the ocular tissues. The company describes ncell as tiny spheres with a hydrophobic core and hydrophilic outer layer which penetrate the aqueous layer of the tear film.
The 12-week, phase 4 multicentre study included 124 subjects who had Cequa twice daily. Evaluating patients’ corneal damage, researchers defined a CFS score of 0 as indicating no stain (healthy cornea) and a score of 4 reflecting a severe stain, before summing all five corneal zone scores. Patients’ mean total CFS score was reported as 5.7 at baseline, which improved significantly (p<0.0001) to 4.0 at week 4, 2.9 at week eight and 2.7 at week 12. The mean mSANDE scores were 67.1 at baseline and improved significantly (p<0.0001) to 48.4 at week four, 44.2 at week eight and 38.3 at week 12, according to Sun Pharma.
Presenting results at the American Academy of Optometry conference, researchers said Cequa was generally well tolerated. Overall, 43.3% of the trial’s subjects reported at least one treatment-emergent adverse event (AE), with 73.8% of those rated mild and the most common being instillation-site irritation and pain. "In addition to the rapid and sustained symptomatic improvement in patients with dry eye disease treated with Cequa, this study is notable for its design, which allows for the use of artificial tears, thus replicating real-world conditions as closely as possible for a controlled clinical trial," said Dr Brittany Mitchell, Sun Pharma’s head of medical affairs, ophthalmics.